Parents and advocates are urging Canada's provinces to work together to make a potentially life-saving drug more accessible to children with spinal muscular atrophy.
"We need all children who are able to benefit from this drug to be able to access this drug," Susi Vander Wyk, of the advocacy group Cure SMA Canada, told CTV News.
Spinal muscular atrophy, or SMA, is a hereditary disease that causes progressive deterioration of muscle strength by damaging motor neurons in the spinal cord. It often leads to paralysis and even death, killing more infants than any other genetic disorder.
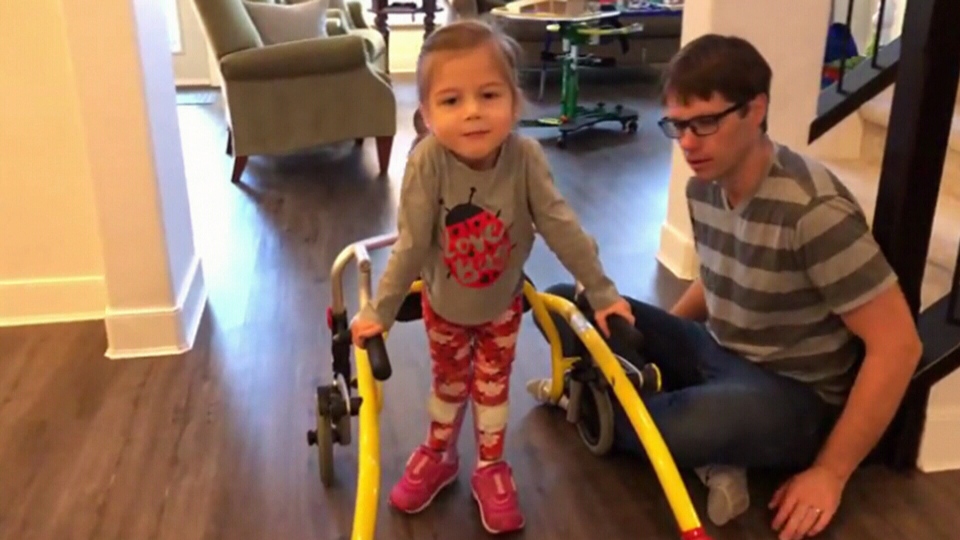
First diagnosed with SMA as a toddler, Natalie Essex was unable to crawl or even sit up on her own for the first few years of her life.
"(It's) just a horrible feeling as a parent to watch this unfold in front of our eyes," her mother said Sunday.
Now four years old, Essex has made remarkable progress and is able to stand with the help of a walker.
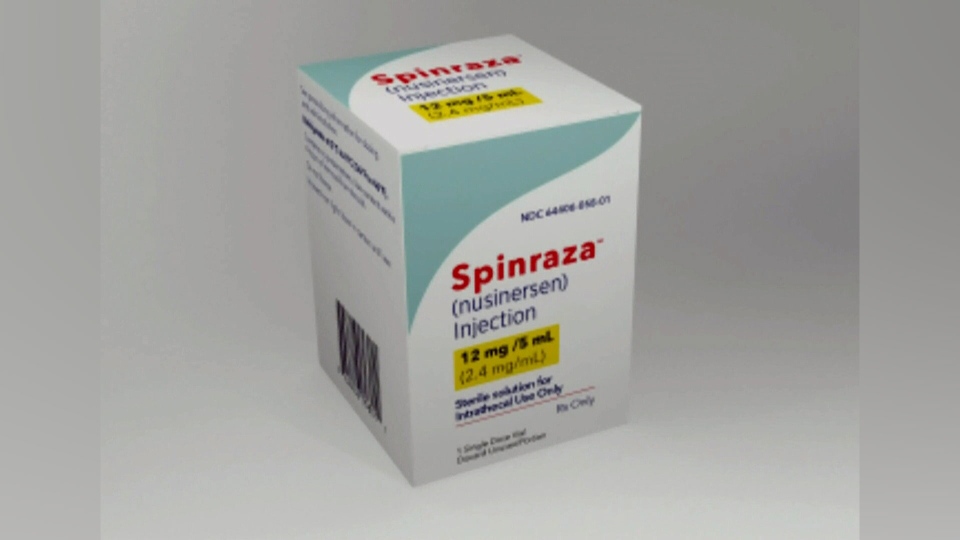
Her recovery is thanks to a drug called nusinersen. Sold under the name Spinraza, the treatment uses synthetic DNA injected into the spinal fluid to promote the production of a muscle-building protein that many people with SMA lack.
"It brings tears to our eyes," said the girl’s father, Andrew Essex. "She knows how well she's doing and I think it makes her proud as well."
Hailed by many as a "miracle drug," Spinraza is proven to halt, and in some cases, reverse the disease in children.
It is also one of the most expensive drugs in the world, priced at about US$750,000 for the first year of treatment alone.
While Health Canada has approved its use in the country, it is only available to a small subset of patients.
In a review of the drug, the Canadian Agency for Drugs and Technologies in Health recommended that nusinersen be refunded for users who have had SMA symptoms for less than 26 weeks and were diagnosed between a week and seven months after birth. These infants, the agency found, were most likely to benefit from Spinraza.
None of the provinces, however, have agreed to fund the treatment.
Children like Natalie have only been able to access Spinraza through a clinical trial that won't last long.
Now, parents say it's up to policymakers to ensure that no children are forced to suffer, even though an effective treatment for SMA is available.
"It's unacceptable that children will die in the process, that children will lose abilities," Vander Wyk said.
The B.C. Ministry of Health would not comment on the issue, other than to say it's currently under review by the Drug Benefit Council, an independent body that advises the government about adding or removing drugs from the PharmaCare program.
Biogen, the company than manufactures Spinraza, told CTV News it plans to work with "health care systems and government to ensure that patients who may benefit…will have access."
With files from CTV Vancouver's Scott Roberts