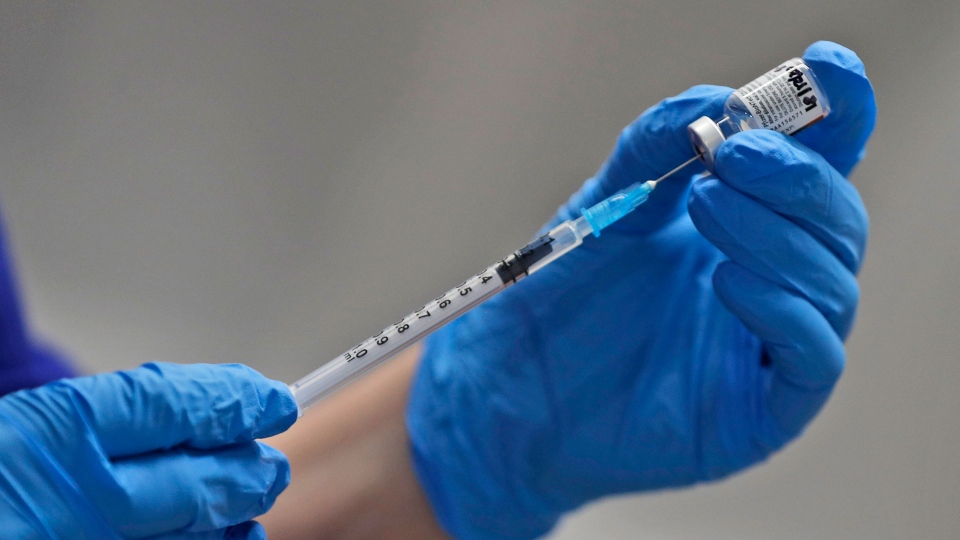
VANCOUVER -- British Columbia's first doses of Pfizer's COVID-19 vaccine will be going to certain frontline health-care workers, including those in the province's long-term care system.
That's partly because of the logistical complications related to Pfizer's vaccine, which has to be kept in extreme sub-zero temperatures and can't be easily delivered directly to seniors' homes and their vulnerable residents.
"The Pfizer vaccine requires us to provide the vaccine at the site where it is delivered," provincial health officer Dr. Bonnie Henry said Wednesday. "That will be the case for the first few weeks of this program, which means we need to bring people to the vaccine instead of the vaccine to the people at this point."
B.C. health officials shared details of their early rollout plans at a briefing with Premier John Horgan, announcing the early doses will be given out at two designated distribution centres equipped with the ultra-low temperature freezers required to store them. Eligible workers will have to go to the sites to receive their doses.
One will be in the Vancouver Coastal Health region and the other will be in the Fraser Health region, though officials have not specified the exact locations.
B.C. is scheduled to receive four trays of about 975 doses each next week, but officials said they expect to receive "tens of thousands" more doses in the last two weeks of December.
They will be distributed to people working in long-term care homes and assisted living facilities, as well as those in high-risk environments such as hospital emergency wards and intensive care units. Henry said immunizing care home workers will help protect the elderly residents who live in the facilities until they can be vaccinated as well.
The decision about who to vaccinate first was made using guidelines set out by the National Advisory Committee on Immunization, which were agreed upon across Canada. Henry said the focus of their decisions has been on saving lives.
"We know the lives that are being taken and affected most are seniors and elders in long-term care and assisted living," she said. "In addition, we have to protect our strained health-care system so all of us can get the care we need, when we need it."
Plans for January and beyond
The province hopes to expand the number of designated distribution centres to nine locations in January, with an eventual eye on setting up dozens in different parts of B.C.
"We're working on having 30 sites around the province that are ready and trained to receive these vaccines and they will have the ultra-low temperature freezers needed on site," Henry said.
Even once the availability of the vaccine is expanded to other groups and demographics, Henry noted the Pfzier vaccine is not approved for everyone. The effects on children under the age of 16, pregnant women and people with immunocompromised conditions are not yet known.
"That is something that we are looking at because we know that people whose immune systems are not functioning – whether that's from cancer treatments or other medications they're on – are at more risk of having severe illness from COVID-19," Henry said. "Unfortunately, we do not yet know if these vaccines work in people who are immunocompromised and if they're safe."
The Moderna vaccine, which officials expect will also receive approval in Canada, is less complicated to transport and Henry said they should be able to deliver that directly to seniors in care. While it must be stored in sub-zero temperatures as well, they aren't as low as those required by the Pfizer vaccine.
Both vaccines require two doses for maximum efficiency.
The B.C. government expects to be able to immunize nearly 400,000 people in British Columbia by the end of March, though officials stressed that's far below what's required for herd immunity.
In the meantime, Premier Horgan noted there's much to be optimistic about after the unexpected challenges the world has faced in 2020.
"We have a glimmer of hope that although the end is not in front of us, the beginning of the end may well be," he said.
"As we see an increase in supply we're going to see an increase in optimism and an increase in opportunities for us to get back to what is remotely resembling normal."
How the vaccines work
Both the Pfizer and Moderna vaccines use a new process called synthetic messenger RNA, which Henry said has been worked on for decades and is now promising a potential revolution in vaccine technology.
"We've taken a piece of the genetic code from the virus that helps translate into the spike protein – that little spikey bit on the outside of the virus," she said. "That protein then is produced by our cells and our immune system responds to that to develop antibodies, and then if we're exposed to the virus, we have those antibodies that attach to it and protect us from becoming ill."
The messenger RNA is a delicate substance that's wrapped in a protective coating called a lipid nanoparticle, Henry said, which is what necessitates the sub-zero storage temperatures.
The rapid development of the vaccines has raised concern among some people, particularly groups who were already prone to vaccine skepticism. Henry noted the quick turnaround was possible, in part, because clinical trials that would normally have happened in sequence were instead conducted simultaneously.
"The greatest brains around the world were put to this process and this task," she said. "And of course we had the addition of a lot of money."
Officials said they were initially hoping for an effective rate of 50 to 60 per cent. Instead, both Pfizer and Moderna have said their vaccines are around 95 per cent effective against the coronavirus.
"The fact that these ones are in the high 90s is the best that we could hope for in public health," Henry said.