Class action lawsuit against Alesse to include those who took the birth control between 2017 and 2019
A national class action lawsuit against the pharmaceutical companies behind Alesse birth control will go forward in B.C. courts.
Two women, Taylor Janet MacKinnon and Alysa McIntosh, who say they got pregnant while taking Alesse, are leading the lawsuit, which was certified as a class action suit by a B.C. supreme court judge this week.
In the documents, both women share their stories of unexpected pregnancies, one having had a miscarriage and the other, who eventually gave birth to a child. They allege the companies were “high-handed, wanton and reckless” by producing and continuing to market a pill that they say was ineffective.
People from across Canada who were prescribed and took Alesse between January 1, 2017 and April 30, 2019 will be allowed to join the lawsuit, which is being stewarded by lawyers at Vancouver firm Rice Harbut Elliott, among others.
Before a class action lawsuit can be heard, a judge must first agree that the case should be heard as a class action suit.
“In this action, the plaintiffs allege that the defendants, Pfizer Canada Inc. and Wyeth Canada, negligently failed to take reasonable steps to ensure that Alesse was safe and effective for its intended use,” reads the June 7 ruling that said the case would proceed.
While the judge’s ruling means the class action lawsuit can proceed and be joined by other Alesse users, the allegations against the drug’s manufacturers have not been proven in court.
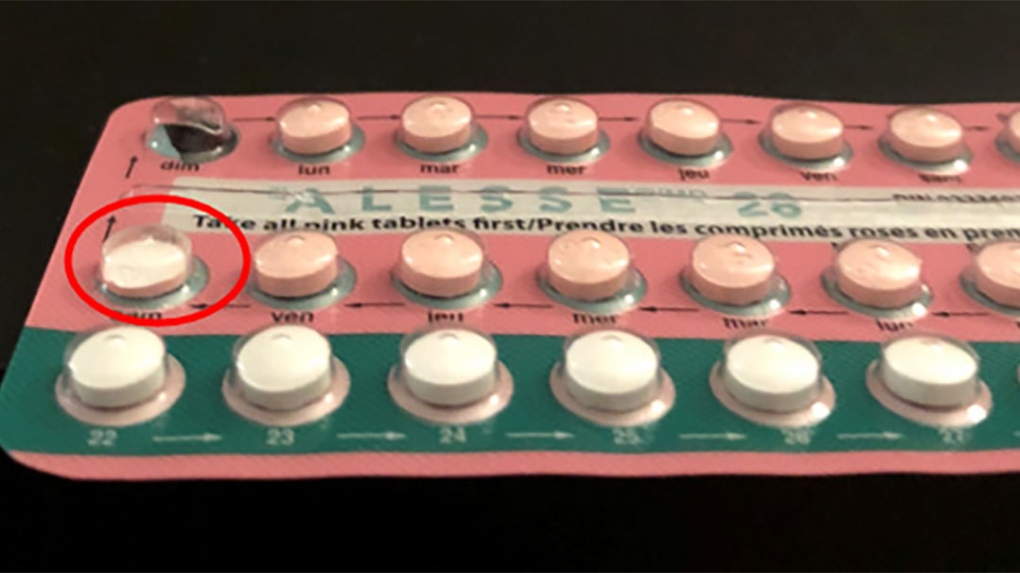
The women say there were “manufacturing defects in Alesse” between 2017 and 2019, which is backed up in part by a Health Canada advisory from December 2017, reads the ruling. The agency told consumers it had received complaints about broken and undersized pills in packages of Alesse 21 and Alesse 28, and issued a warning.
MacKinnon had been taking Alesse for several years to prevent pregnancy, and says she always took them as directed. At the beginning of December 2017, her pharmacist advised her of the recall and told her to check her pills for abnormalities. However, about 10 days later she discovered that she was already five weeks pregnant.
MacKinnon alleges she contacted Health Canada and Pfizer to alert them about what happened, but that neither provided her with much information.
She gave birth to a daughter about nine months later. She was 24 at the time of the birth, and says the early and unexpected pregnancy caused both financial and career instability.
“MacKinnon says she wished to have children someday but not at such a young age,” reads the judge’s ruling.
“She would have preferred that she and her partner were more established in their careers and financially stable before having children … she has not been able to find work as a certified dental assistant following the birth of her daughter.”
Meanwhile, McIntosh, who had been taking Alesse for about 11 months -- also as directed -- discovered she was pregnant at the end of October 2017, only to have a miscarriage near the beginning of December, when the fetus was about nine weeks old.
Lawyers had packages of Alesse tested by a lab, which found that some of the pills did not contain the amount of estrogen that was advertised on the packaging. The pills tested weren’t even the visibly broken or damaged pills that Health Canada had warned of.
“The plaintiffs say that an inference can be drawn that estrogen levels would be even lower in chipped or broken pills … (and the) testing demonstrates issues with estrogen levels that go beyond broken or chipped pills,” says the ruling.
According to the ruling, lawyers say that 138 people have contacted them to be part of the case, and Health Canada has a record of 38 people who had issues with the birth control. The overlap between the two lists is not yet known, and notice of the class action case has not yet been widely circulated.
Both women say the companies were negligent and are seeking financial damages. They also allege that by continuing to market Alesse despite the recalls and known issues, the companies deceived and misled consumers.
But the companies deny any wrongdoing. While the complainants will rely on three expert witnesses who say the lower levels of estrogen were likely to reduce the effectiveness of Alesse at preventing pregnancy, an expert witness for the companies disagrees. According to the ruling, that expert will argue that the reduced amount of estrogen in the pills was “within an acceptable range of deviation” that has “no impact on pregnancy rates.”
The companies also argue that the amount of estrogen in their pills was within a range accepted by Health Canada.
The judge notes that the women and others who apply to be part of the class action case may have difficulty proving that the pills had reduced effectiveness, but said they presented enough information to be certified and allowed to proceed with their case.
Provincial health-care services from across the country are also joining the class action case, and will be seeking to recover the health-care costs associated with what they say was negligence from Pfizer and Wyeth.
CTVNews.ca Top Stories

Quebec nurse had to clean up after husband's death in Montreal hospital
On a night she should have been mourning, a nurse from Quebec's Laurentians region says she was forced to clean up her husband after he died at a hospital in Montreal.
Northern Ont. lawyer who abandoned clients in child protection cases disbarred
A North Bay, Ont., lawyer who abandoned 15 clients – many of them child protection cases – has lost his licence to practise law.
Bank of Canada officials split on when to start cutting interest rates
Members of the Bank of Canada's governing council were split on how long the central bank should wait before it starts cutting interest rates when they met earlier this month.
Maple Leafs fall to Bruins in Game 3, trail series 2-1
Brad Marchand scored twice, including the winner in the third period, and added an assist as the Boston Bruins downed the Toronto Maple Leafs 4-2 to take a 2-1 lead in their first-round playoff series Wednesday
Cuban government apologizes to Montreal-area family after delivering wrong body
Cuba's foreign affairs minister has apologized to a Montreal-area family after they were sent the wrong body following the death of a loved one.
'It was instant karma': Viral video captures failed theft attempt in Nanaimo, B.C.
Mounties in Nanaimo, B.C., say two late-night revellers are lucky their allegedly drunken antics weren't reported to police after security cameras captured the men trying to steal a heavy sign from a downtown business.
What is changing about Canada's capital gains tax and how does it impact me?
The federal government's proposed change to capital gains taxation is expected to increase taxes on investments and mainly affect wealthy Canadians and businesses. Here's what you need to know about the move.
New Indigenous loan guarantee program a 'really big deal,' Freeland says at Toronto conference
Canada's Deputy Prime Minister Chrystia Freeland was among the 1,700 delegates attending the two-day First Nations Major Projects Coalition (FNMPC) conference that concluded Tuesday in Toronto.
'Life was not fair to him': Daughter of N.B. man exonerated of murder remembers him as a kind soul
The daughter of a New Brunswick man recently exonerated from murder, is remembering her father as somebody who, despite a wrongful conviction, never became bitter or angry.